BioMed Revision: CHEMISTRY

Disclaimer
Whilst some care has been taken to check externally linked websites no responsibility is offered nor implied for the suitability, legality or reliability of content therein.
Prof Dave is full of helpful answers: All his playlists
Just this theme: General Chemistry
2.1 Where & Why The Electrons Group
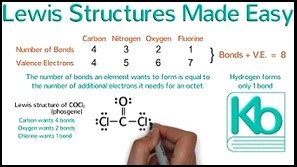
Drawing Dot structures -easy stuff
- Prof Dave 7m26
2. Atomic Structure
2.2 Electronegativity Decides On Bonding Type
What makes a bond be Covalent or Ionic, Polar or Nonpolar - Prof Dave 3m32
2.3 Explaining The s,p,d,f Sublevels Per Shell
Energy Levels, Energy Sublevels, Orbitals, & Pauli Exclusion Principle - Richard Louie 12m09
1. Atoms
Useful things not part of the course..
More stuff to come here as needed..
3. Future subjects here
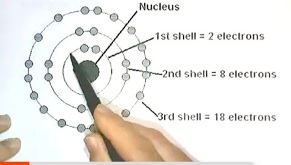
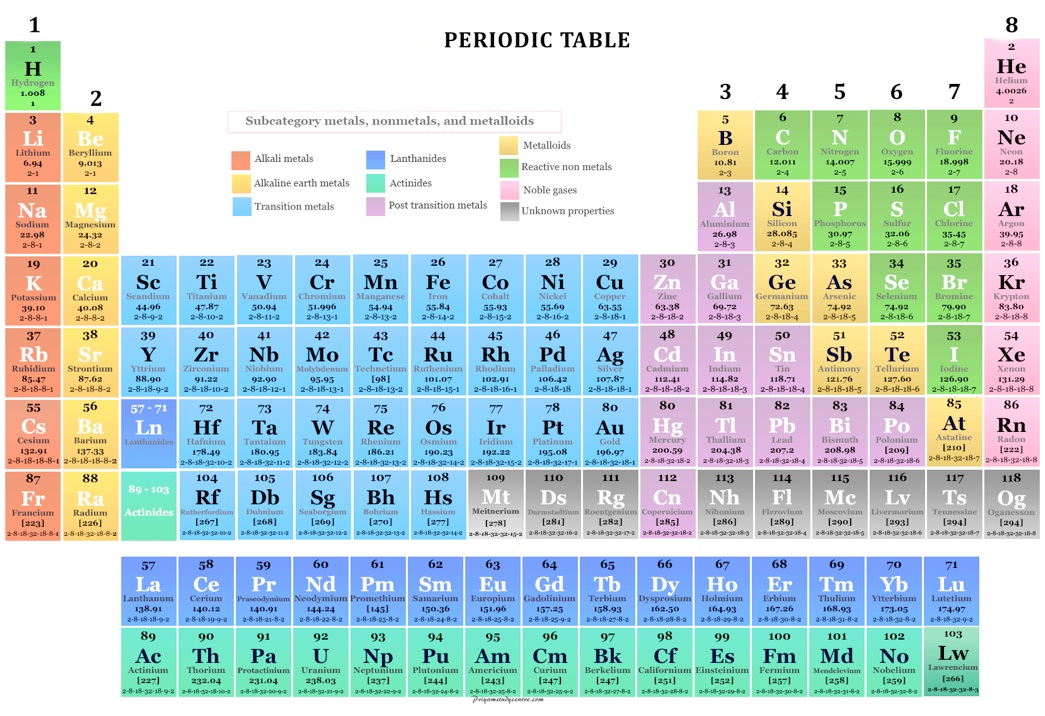
1.1 Periodic Table
Includes numbers of electrons per shell
1.2 A Better Way To Picture Atoms
All these little sun-and-planet diagrams are poor at conveying the regions where an electron is most likely to be found. These graphics are stunning..
- Henry Reich for Minute Physics 5m34
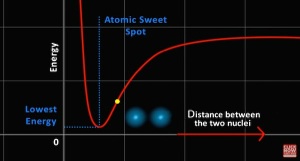
1.3 Why Atoms Form Molecules - simply
Even though clouds of electrons repel each other AND the nucleii repel each other the electron - proton charge attraction gives better entropy.
- Arvin Ash 13m24
Arvin Ash has a massive list of videos.
See here for the atomic ones.. The Universe
Last update: 25/06/2023
For Later
Helpful description here: BiologyOnline: Active Transport
In reverse chronological order..