Fascinating Chemistry

A. Periodic Table
Disclaimer
Whilst some care has been taken to check externally linked websites no responsibility is offered nor implied for the suitability, legality or reliability of content therein.

Since the very dawn of man’s understanding of the world we have sought to build up and break down the materials that we see around us. After the physical breaking down of rocks and plants man most likely burnt materials. This process can be partial or complete but when burning wood, in either case, often carbon would be left. Unknowingly then this was then the first elemental substance to be identified. Since then man has sought to categorise substances and some just cannot be isolated any further. We call these elements and it is only since trying to purify metals was any form of rigor applied to the subject.
This was the dawn of the science of chemistry. Slowly people realised that there were patterns to the way that elemental substances reacted with one-another. Starting in 1661, with Robert Boyle stating that elements are "those primitive and simple bodies of which the mixt ones are said to be composed and into which they are ultimately resolved”, it took two further centuries of many different kinds of discoveries before John Newlands Law Of Chemical Octaves came in 1864 and finally Dmitry Mendeleyev proposed the Periodic Table in 1868.
The map was of course incomplete but it gave chemists a far better guide of where to look for the missing pieces to a jigsaw that these days we now take for granted. It was a major leap forward and is still the best way of using the overlapping subjects of physics and chemistry to explain the organic and inorganic world in which we live. The most common one are in this interactive table below -the gaps are not missing merely omitted for clarity here.

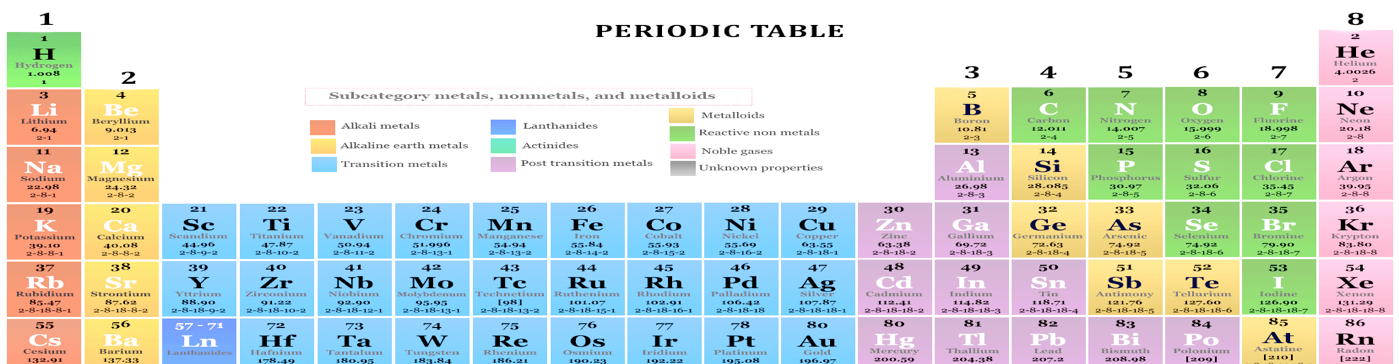
A much more detailed version can be found here: Periodic Table

Li
Lithium
Na
Sodium

Rb
Rubidium
Cs
Caesium
Be
Berylium
Mg
Magnesium
Ca
Calcium
Sr
Strontium
Ba
Barium
Cr
Chromium
Mn
Manganese
Fe
Iron
Co
Cobalt
Ni
Nickel
Cu
Copper
Pa
Palladium
Pt
Platinum
Ag
Silver
Au
Gold
Zn
Zinc
Cd
Cadmium
Hg
Mercury
Al
Aluminium
Sn
Tin
Pb
Lead
B
Boron
Si
Silicon
Ge
Germanium
C
Carbon
N
Nitrogen
P
Phosphorus
O
Oxygen
S
Sulphur
F
Flourine
Cl
Chlorine
Br
Bromine
I
Iodine
He
Helium
Ne
Neon
Ar
Argon
History
The history of chemical discovery is an amazing story in itself. Wikipedia have done an excellent job: History Of The Periodic Table
Fascinating Chemical Facts
Some of the elements named above are highlighted in blue. These link to fascinating stories often more than just the history of the substance named.
(There are only a few at present. They are good and more will be added as time goes on so do come back and check the Last Update date at the top right)
Detailed Data
Extremely detailed data on all elements, including physical/electronic properties, etymology and history:
See: Chemicool
Plus energy levels: PTable.com
But for a really superb rundown on the properties of every single element see the periodic table at Royal Society of Chemistry. Hover over for a quick glacnce or click to go to a full webpage. At the top, in rather small writing you can move forward and back (by Atomic Number). On each page there is a link to ChemSpider -click the Articles tab and you will find a list of links and abstracts about the isotopes and uses of that element. The list can be long in some circumstances.
Goto: Interactive Periodic Table: Royal Society of Chemistry
B. Colour
Colour In Our World
Most mammals see our world in colour. That colour is there both naturally and by biological means. This is a superb website with a search function. Particularly fascinating is the study of Chlorophyl.
Further Information
Scroll down on the Fascinating Biology page to: What Animals See In Colour Or At Night
Flame Tests
If atoms are made more excited, usually by heating but it can be useful in electronics eg TVs, when the atom drops back into its unexcited state nanoseconds later it releases its energy dependent on the size of the gap between energy levels which is fixed per element. Quite a few elements do this in the visible spectrum. These colours can be seen by exposing a compound, containing the following metals, to the extremes of heat in a suitable flame eg bunsen burner. The the colours seen are shown..
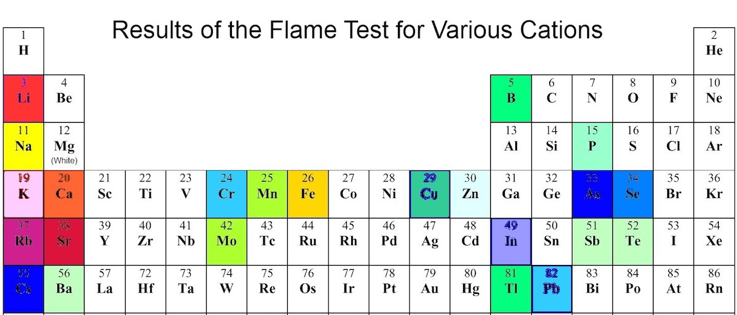
Note this electronic Spectroscopy of quite different to physical Chromatography.
E. Acids & Alkalis
A-Level Help
The following file was part of the British A-Level syllabus. It starts off easy and works up to the best explanations and examples that I’ve ever seen (2Mb download): AcidAndBaseTheoryComplete_AdvancedLevel.pdf.
I don’t like this website anymore. I used to be well laid out but it isn’t anymore. However it does give useful explanations: LibreTexts: Overview of Acids & Bases
Strengths relative to pure water.
Last update: 23/09/2024

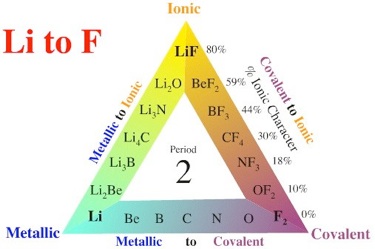
C. Bonding Between Atoms
Three Types
There are three main types of bonding. This triangle uses the extremes of Lithium and Flourine to illustrate the relative strengths of bonding in simple compounds.
Be aware that this does not cover all elemental self bonding, that of large molecules and temporary biological processes. These are covered here (0.8Mb download):
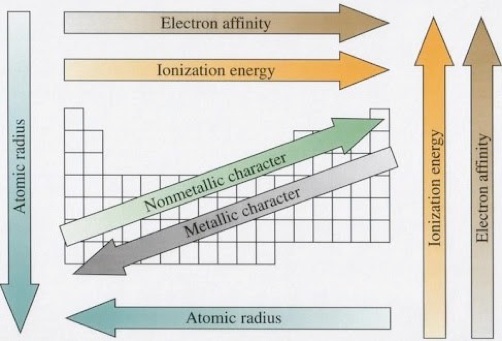
D. Electronegativity
GCSE Help
In metals electrons are completely free of their parent atoms. Bonding occurs within this sea and thus metals have many useful properties like malleability, electron conduction etc.
Covalent and Ionic bonding involves the restricted sharing of electrons -but rarely equally.
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons.
Periodic table showing relative Electronegativity (Pauling Scale).
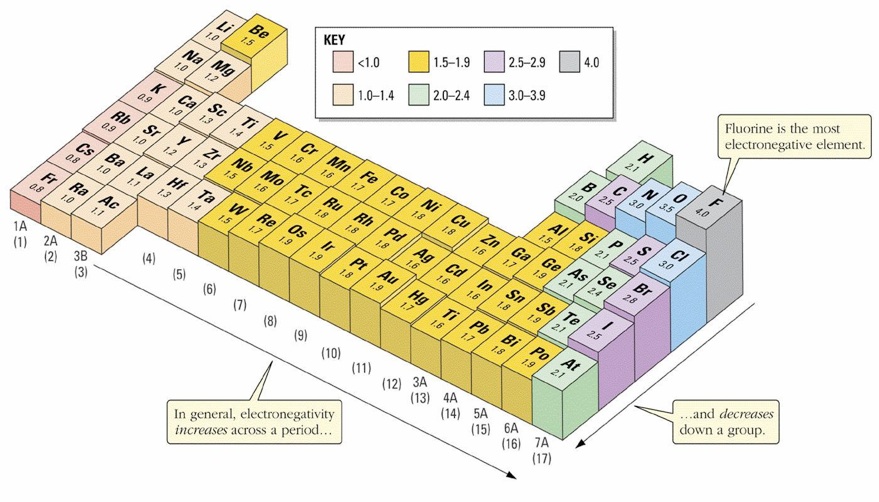
The implication of all this is that there is no clear-cut division between covalent and ionic bonds. In a pure covalent bond the electrons are held on average exactly half way between the atoms. In a polar bond the electrons have been dragged slightly towards one end.
How far does this dragging have to go before the bond counts as ionic? Whilst there is no real answer normally sodium chloride is considered as being a typically ionic solid. However even here the sodium hasn't completely lost control of its electron. Because of the properties of sodium chloride we tend to count it as if it were purely ionic.
See here for more: ChemGuide: Electronegativity
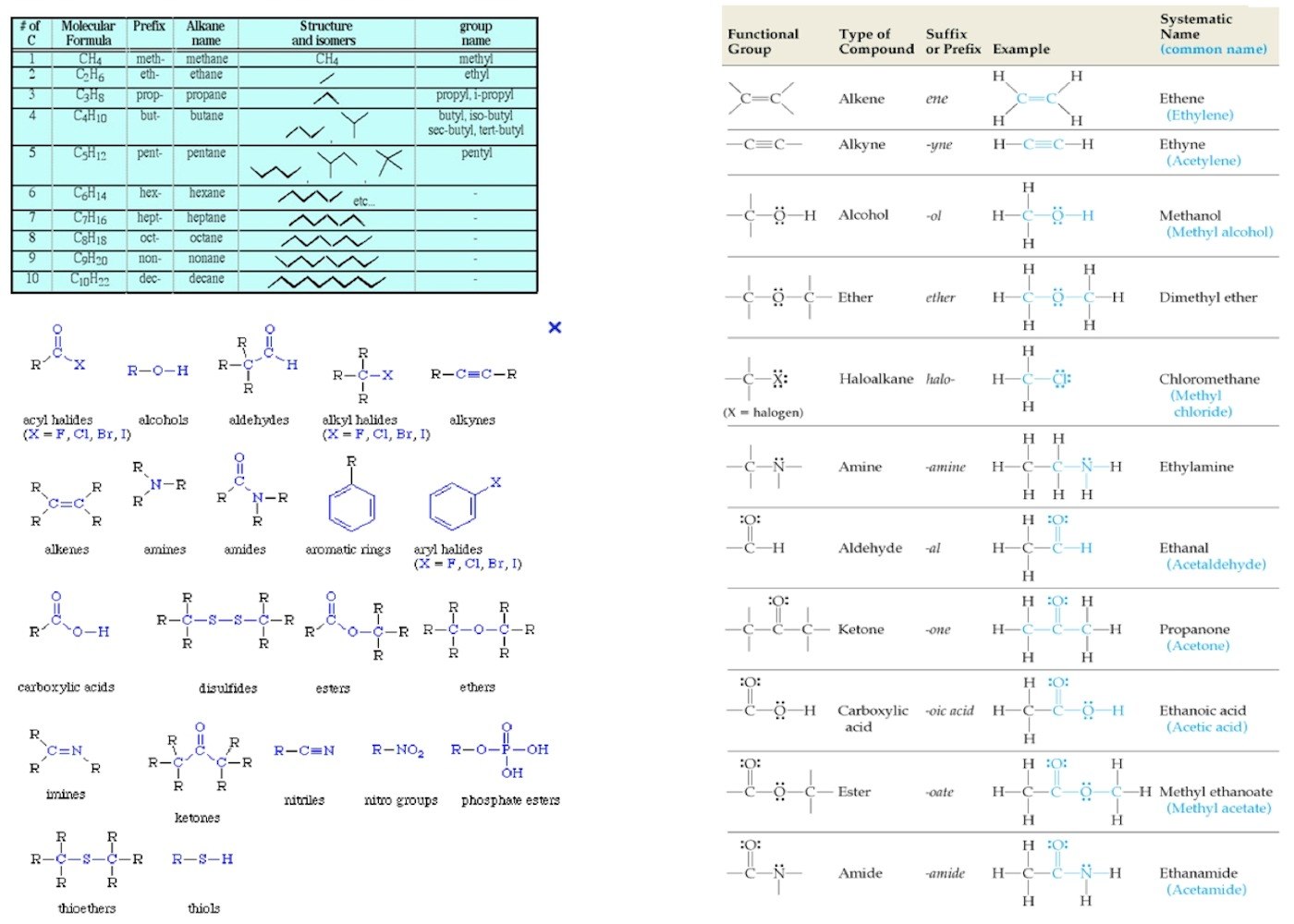
F. Organic Chemistry
This is my very favourite area of chemistry but it’s not for everyone so I merely append a chart of modern naming conventions here. As I said on the Need A Website To.. page the Khan Accademy gives free courses on a wide range of subjects. For help you could do worse than to see the free short video tutorials and discussions available here: Khan Accademy: Organic Chemistry